SERVE-HF and ADVENT-HF A Brief History and Summary
Contents
Introduction
A brief background on the current warning for individuals with heart failure and a left ventricular ejection fraction less than 45% (LVEF < 45%). The SERVE-HF study published in 2015 and enrolled in 2012 used older style ASV machines with fixed EPAP pressure on patients that were not titrated, or monitored for compliance. An unexplained but statistically significant risk of death from cardiac failure was found for a cohort that had severe heart failure and very low ejection fraction. Although the cause of these deaths was not identified, the study was discontinued and a recommendation to avoid ASV in heart failure patients with LVEF < 45% was issued. This warning was conservatively formulated, but fear of causing harm in patients led to a generalized rejection of ASV therapy for anyone, and we saw a return to ST machines. Logically, where ASV is needed, the patients unable to be properly treated experienced deteriorating health and death from failing to treat their Cheyne-Stokes Respiration, central apnea and other significant respiratory problems.
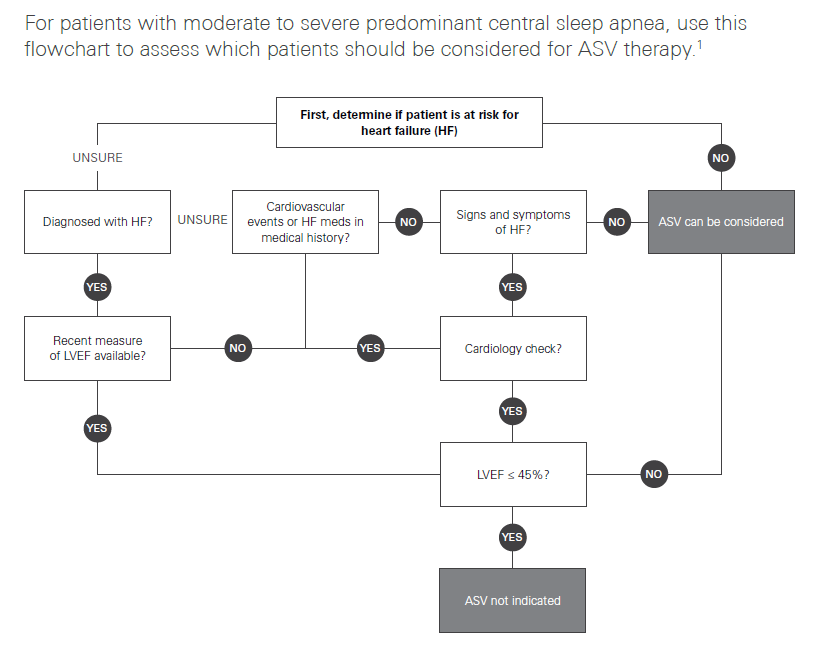
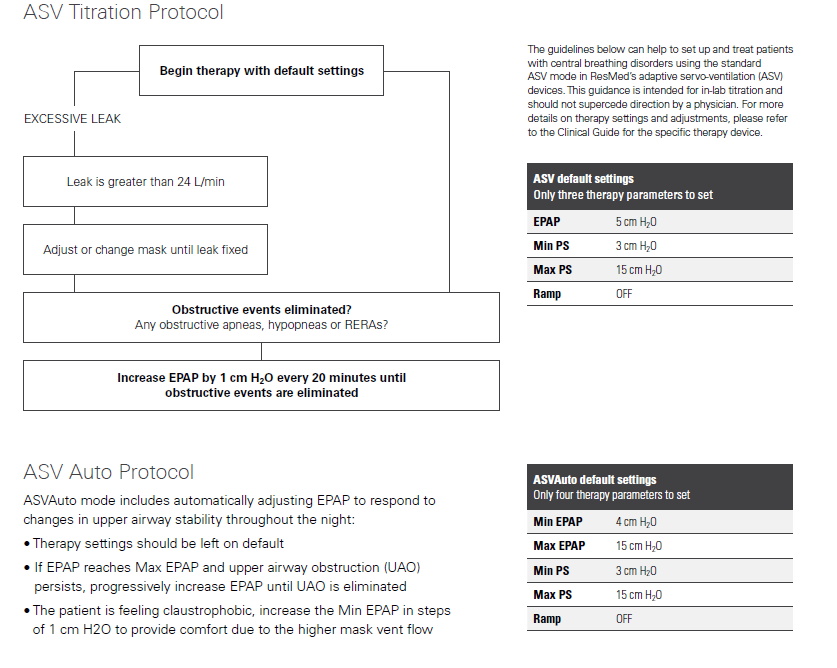
A new study was enrolled called ADVENT-HF which used more contemporary machines with auto-adjusting EPAP, titrated patients monitored for compliance. That study failed to replicated the deaths or any severe illness in the cohort of severe HF patients with low LVEF. I expected that the study would be published this past year putting an end to the ASV nonsense that we have been dealing with for the past five years, but we're still waiting. It needs to be clearly understood, that the "warning" for ASV and requirement for echocardiogram testing is ONLY applicable to heart failure patients with risk factors consistent with the SERVE-HF study, not to healthy individuals like you. Individuals with normal heart function or no known risk factors can move directly to titration and use for ASV. Out of an abundance of caution you may be asked to take the heart function tests, but it is really not necessary nor part of the guidance. This is the Resmed flow-chart on the matter, followed by the ASV titration procedure. While we are not practicing medical professionals on the forum, we generally take the position that in cases like yours, the use of clinical titration testing is unnecessary for someone that shows a need for ASV due to complex or central apnea without complicating pulmonary disease because the auto ASV algorithm automatically resolves both obstructive and central apnea without any input from a technician. This is a major advancement over the older machines that did not have the auto-adjusting EPAP as mentioned above under the SERVE-HF discussion. It is sufficient to allow the patient to use ASV using default ASV Auto settings and determine optimization as needed, and establish patient benefit to the therapy after the trial.
Resmed Clinical Screening Guideline for ASV
Resmed Clinical Titration Guideline for ASV
SERVE-HF
Great attention has been paid to the SERVE-HF trial (“Treatment of Sleep-disordered Breathing with Predominant Central Sleep Apnea by Adaptive Servo Ventilation in Patients with Heart Failure”), which showed increased all-cause mortality and cardiovascular mortality in the Adaptive Servo-ventilation (ASV) group compared with the control group of conventional heart failure management alone. The results of this trial led to the recommendation by multiple ASV manufacturers and medical societies to withdraw clinical use of ASV from patients with heart failure and a reduced ejection fraction (HFrEF) less than 45%.
SERVE-HF was the first randomized, large scale, multinational trial directed to treat CSA in patients with HFrEF < 45% and concomitant clinically significant sleep apnea with AHI > 15/hour of central predominance (CSA index >10/hour). Treatment arms compared the addition the ASV, one of the most effective noninvasive positive pressure ventilation technologies for central apneas that offers automated inspiratory pressure support in addition to expiratory positive pressure vs conventional medical treatment alone in the control group. (Lessons Learned SERVE-HF, 2017)
The study published in the New England Journal of Medicine in September 2015 was designed in an intention-to-treat basis with the primary end point of time to first event, a composite of death from any cause, lifesaving cardiovascular intervention (heart transplant, implantation of LVAD, resuscitation after sudden cardiac arrest, or defibrillation for ventricular arrhythmia), or unplanned hospitalization for heart failure. The study did not show significant differences in the primary end point between the ASV and control group (54.1% and 50.8%, respectively; hazard ratio, 1.13; 95% confidence interval, 0.97 to 1.31; P=.10).1
The most interesting and unexpected outcome was an increase in the all cause mortality and cardiovascular mortality in the ASV group (hazard ratio for death from any cause, 1.28; 95% CI, 1.06 to 1.55; P=.01; and hazard ratio from cardiovascular death, 1.34; 95% CI, 1.09 to 1.65; P=.006).1 These findings led to the above recommendations from manufacturers, as well as a position statement from the American Academy of Sleep Medicine. These findings cannot be extrapolated to the obstructive sleep apnea population with concomitant HFrEF or to patients with HF with preserved ejection fraction, where positive pressure ventilation has offered an advantage1 likely by a different physiologic mechanism not fully uncovered at this time, believed to be an overall effect of afterload reduction.
Selection and self-selection bias in this study was addressed in a new analysis by the same SERVE-HF investigator group published August 2017, where a time-dependent model of on-treatment analysis (done to tease out if the original results were related to the treatment assignment or to poor adherence) was conducted to understand potential causes of the initial findings in the original study. It showed patients randomized to ASV who crossed over to the control group were at higher risk of cardiovascular death than control subjects; also the control patients with crossover to ASV had a signal of lower risk of cardiovascular death risk compared with patients assigned to ASV.2 Reduced adherence to ASV treatment during SERVE-HF was a concern, since it resulted in a reduced exposure to the treatment. The on-treatment analysis showed again an increase of cardiovascular death in HFrEF patients with predominant CSA treated with ASV in addition to conventional heart failure treatment compared with the control group.2 There was no increase in cardiovascular death risk associated with ASV use intervals (dose effect). This effect is not related to the amount of hours used per night.
The effect of the recommended withdrawal of treatment in HFrEF patients with EF<45% and moderate to severe central predominant sleep apnea is being addressed in smaller studies. A single center retrospective analysis observed the effects after ASV discontinuation in this population. Thirteen out of 126 patients treated with ASV who met SERVE-HF criteria were followed for at least a year; 93% of the subjects who met criteria had ASV removed; immediate recurrence of the central apnea was observed in most (except two patients), while adverse events were not identified (defined as need for emergency hospitalization). Day and nighttime symptoms were reported by 61% of the group, and they were started on alternative treatments.3 At 1 year after ASV removal, 88% of patients were still alive, overall cardiac function did not change in 1 year (P=0.17), and seven patients required adjustment of medications for heart failure. Symptomatic patients were treated with oxygen supplementation for nocturnal symptoms or CPAP if they had daytime sleepiness. None was treated with bi-level PAP, acetazolamide, or phrenic nerve stimulation. Four patients insisted on continuation of ASV despite understanding physician concerns. 3 This study helps to demonstrate that ASV discontinuation is feasible but requires close follow-up. However, larger, long-term prospective reviews are required to draw statistically meaningful conclusions about the consequences and safety of ASV removal; these studies will be difficult to conduct under the current indications for ASV in the interest group.
At this time, investigators have shifted to further understand the causes of the increase in cardiovascular mortality, overall mortality, and the understanding of the pathophysiologic processes associated with ASV use in HFrEF. It is not known whether the effect in mortality is related to the specific ASV device/algorithm used to suppress CSA or is related to the ASV principle itself. Upcoming studies will assist in clarifying these details. Currently, there is an ongoing trial looking at the effect of ASV on survival and hospital admissions in heart failure (ADVENT–HF) using a different ASV device; this study will hopefully elucidate the impact of class effect vs device effect. It may also provide better insight of the etiology of mortality and the impact of improved ASV compliance, first addressed by the on-treatment analysis of the SERVE-HF.4
Although the reasons for increased mortality related to ASV are unclear, proposed hypotheses include: central apnea is an adaptive mechanism to HFrEF and the reversal of central apneas might adversely affect the underlying disease process,1 low adherence to ASV may impact outcomes, and specific devices may induce hyper-/hypoventilation generated by the algorithm designs of the specific ASV device and this may result in electrolyte abnormalities that generate arrhythmias.
Analysis of SERVE-HF Study Flaws
SERVE-HF More Questions Than Answers The recent publication of SERVE-HF, a trial that evaluated the effect of treating Hunter-Cheyne-Stokes breathing with central sleep apnea (CSA) in patients with heart failure and reduced ejection fraction (HFrEF), has raised serious concerns about the safety of adaptive servoventilation (ASV) in this population.1 In addition to having no effect on the primary composite end point, post hoc analysis revealed an unexpected association between randomization to ASV and excess cardiovascular mortality. The investigators speculated that (1) CSA might represent a compensatory mechanism positive-airway pressure therapy randomized controlled trial (CANPAP) suggested that when CSA is effectively treated, survival improves.5 Our careful reading of the paper,1 its online supplement, and the study protocol6 revealed important design, data collection, and analysis concerns. We address some of these issues below
ADVENT-HF
The ADVENT-HF trial, designed to test whether adaptive servo ventilation (ASV) improves cardiovascular outcomes in heart failure patients with sleep apnea, so far has better compliance than previous trials, with no safety concerns to date, according to investigator T. ADVENT-HF Early Results: High ASV Compliance, No Safety Concerns As of 2018, the study had been reviewed five times by the data safety monitoring board since the announcement of SERVE-HF trial results, with no safety concerns in either obstructive sleep apnea or central sleep apnea patients.
The ADVENT-HF trial, although similar in design, has significant differences from SERVE-HF: different ASV devices may have a different impact on cardiac output and ventilation, recruited patients included those with less daytime sleepiness, and the potential to assess the effect of ASV in patients with OSA and low daytime sleepiness in patients with reduced EF.5,6 This ongoing study may help us to further understand why there is increased mortality and what effect ASV has on the treatment of sleep apnea in patients with HFrEF. with protective effects in HFrEF patients and (2) excess positive intrathoracic pressure caused by ASV might have had adverse cardiovascular consequences.
In our opinion, the former hypothesis has little scientific basis and lacks experimental support. In contrast, much experimental evidence exists that hypoxia, arousals, and increased sympathetic activity consequent to CSA have adverse cardiovascular effects that are reversed with positive-airway pressure therapy.2-4 In addition, the post hoc analysis of the Canadian continuous

Donate to Apnea Board